Introduction
The connection between pavement and pollution may not be immediately obvious. However, the United States has nearly four million miles of roads. (Low Impact Development Center, Inc. et. al.[1]) In a very simple breakdown, people drive and goods are transported on these roads; burning of fossil fuels in engines creates air pollution, while rain and engine leaks create runoff.
Stormwater Runoff
Stormwater management reduces the impact development has on the local landscape. This includes resource protection, water recharge, and also may include any environmental codes or licensing required by the government. The goals of controlling stormwater include reducing runoff rate, improving runoff clarity, increasing infiltration, reducing water pollution, and improving evaporation. Water quality is a major goal, as well as stabilizing stream flow-rates in the area. (Low Impact Development Center, Inc. et. al.[1])
Turbidity
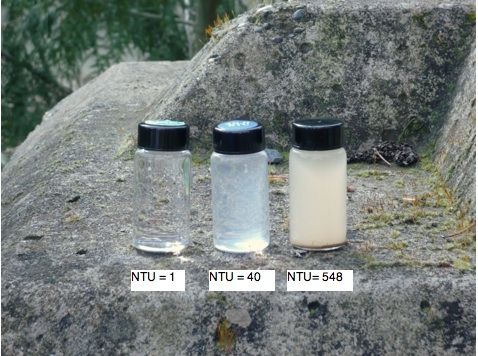
Turbidity is a measurement of how murky the water is, in other words, how much light is transmitted through the items. Turbidity may be caused by clay, algae, or other organic or inorganic matter, in other words, the suspended solids. Tiny particles make the water look unpleasant, can damage fish habitat, and also create difficulties when removing or sanitizing pathogens. (Weiner and Matthews, 2003[2])
Turbidity is measured by a turbidimeter, a device which measures the intensity of light scattering. The amount of scattered light is an indication of the turbidity, in that opaque particles will scatter the light. (Weiner and Matthews, 2003[2]) The measurement unit for turbidity is Nephelometric Turbidity Units, or NTUs.

Material can be found in stormwater runoff, as previously mentioned. This material is categorized as the total solids. Total solids can be further broken down into total dissolved solids, which is any dissolved or colloidal material that passes through a 2.0 micrometer filter. The solids that are retained on this filter are the total suspended solids. (Weiner and Matthews, 2003[2])
Oil Sheen
Oil sheen is the rainbow effect on the surface of water that indicates release of a petroleum product. Petroleum products are toxic to fish and plants, and can be difficult to remedy upon release.
|
|
|
Nitrogen and Phosphorous
Nitrogen and phosphorous can both be described as limiting nutrients in a system. Excess nutrients promote eutrophication. Simply, eutrophication is the loss of oxygen from a lake, due to the death and decomposition of algae. Bacteria use oxygen to convert algae into a carbon source. The loss of this oxygen from standing water, as in a lake, creates conditions that prevent aerobic life from continuing. It is in the best interests of the responsible engineer or contractor to prevent the loss of life and productivity due to excess nutrients in water. Phosphorous tends to adhere to inorganic materials and is common in the sediment from stormwater runoff. Nitrogen is typically leached from soil or moves with organic matter, and is a common fertilizer. (Weiner and Matthews, 2003[2])
pH
PH is the measure of acidity or alkalinity of a liquid. It has no units and is typically expressed in whole or decimal numbers. Low pH indicates acid solutions, while a high pH means the solution is basic. A pH of 7 is considered neutral. An acceptable pH for drinking water is in the range of 6.5 to 9.5. For each whole number change, the pH changes by a factor of ten. This means that pH 6 is 10 times more acidic than pH 7. Water’s pH is important since life cannot function at a pH too acidic or basic.
Air Pollution
The World Health Organization lists several gasses as criteria pollutants. These are common air pollutants, and may cause health issues, damage the environment, and may damage property. The criteria air pollutants are: Carbon monoxide, lead, nitrogen dioxide, ozone, PM10 and sulfur dioxide. Some of these have been mentioned previously. Notably, cement production releases particulate matter, CO2 and other gasses. (Greenway, 2003[3])
Carbon Dioxide
Cement production is a well-known and researched issue contributing to CO2 production. As of 2000, approximately 3.4% of global CO2 production was due to cement production processes. (Hanle, Lisa J.[4]) Cement production relies heavily on fossil fuels, such as coal and petroleum coke, in order to heat the clinker to the high temperatures (approximately 1500 degrees Celsius) necessary to create it from limestone.
CO2 is released from two processes in cement production: heating the kilns to produce klinker, and a reaction that converts limestone to CaO and CO2. The chemical reaction produces CO2 in proportion to the lime content of the clinker. (Hanle, Lisa J.[4])
Particulate Matter
Particulate matter is measured in total and as particles of a specific size range. First is the portion of particles under ten micrometers, or PM10 and second, the particles less than 2.5 micrometers in size, or PM2.5. Short term evidence based on several studies suggests that for every increment increase of 10 µg/m3 in PM10, there is an increase of about 0.5% in daily mortality. By increasing the short term (24 hour) standard from say 50 to 150 µg/m3, the daily mortality will increase by 5%.
Long term evidence suggests that there is about a 15% higher long-term mortality risk when comparing PM2.5 levels of 10 versus 35 µg/m3. This amounts to an increase of a bit more than a 0.5% increase in mortality for every 1 µg/m3 increase in PM2.5. (WHO, 2005[5])
Other Gasses
The table illustrates some of the WHO guidelines for some common air pollutants which have not been specially mentioned.
| Measurement | Ozone (µg/m3) | NO2 (µg/m3) | SO2(µg/m3) |
|---|---|---|---|
| Various sampling periods | 100 (8-hour mean) | 40 (annual mean) | 20 (24 hour mean) |
| — | 200 (1 hour mean) | 500 (10 minute mean) |
There are both long and short term evidence to support these guidelines (like those for particulate measures such as PM10 and PM2.5).
- Evaluation of Best Management Practices for Highway Runoff Control: Low Impact Development Design Manual for Highway Runoff Control (LID Design Manual)↵
- Environmental Engineering 4th Edition. Butterworth Heinemann. Burlington, MA.↵
- How to Obtain Air Quality Permits. McGraw-Hill. New York.↵
- CO2 Emissions Profile of the U.S. Cement Industry. US EPA. Washington, D.C.↵
- ”WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide, and Sulfur Dioxide—Summary of Risk Assessment,” 2005.↵


